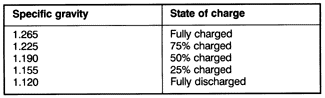
Hydrometer TestingThe hydrometer consists of a glass cylinder with a freely moving float inside. When electrolyte is drawn into the cylinder, the level to which the float sinks indicates the specific gravity of the electrolyte. The more dense the concentration of sulfuric acid in the electrolyte, the less the float will sink, resulting in a higher reading (and state of charge). Electrolyte temperature affects the reading, so a thermometer should be used to determine electrolyte temperature before making a hydrometer test. Add .004 to the hydrometer reading for every 10°F (6°C) that the electrolyte is above 80°F (27°C); (80°F); subtract .004 from the reading for every 10°F (6°C) that the electrolyte is below 80°F (27°C). Before checking the specific gravity of a battery, load the battery with 15 amps for one minute. This can be done by turning on the headlights without the engine running if the battery is installed in the vehicle. Table c lists the percent of charge based on specific gravity values. Table c. Specific Gravity of Electrolyte at 80°F (27°C)
The battery is in satisfactory condition if the average specific gravity of the six cells is at least 1.225. If the specific gravity is above this level, but the battery lacks power for starting, determine the battery's service condition with a load voltage test, as described below. If the average specific gravity of the six cells is below 1.225, recharge the battery. If, after recharging, the specific gravity varies by more than .050 between any two cells, replace the battery. |